By Science and Technology Office | Updated: 2024-11-05
Recently, Associate Professor Jin Hanyong from the School of Pharmacy at Yanbian University achieved an important breakthrough in revealing the biogenesis mechanism of transfer RNA (tRNA) fragments and how cells use tRNA fragments to cope with endoplasmic reticulum (ER) stress. He published his latest research findings as the first author in Nature Communications. This study delves into the process by which the IRE1α enzyme generates specific tRNA fragments under ER stress conditions and their crucial role in cancer cell proliferation and adaptation, offering new insights into cancer biology and cellular stress responses.
In recent years, tRNA-derived fragments (tRFs or tsRNAs) have attracted increasing attention as a novel type of non-coding RNA. Their potential regulatory functions in cancer, metabolic disorders, and epigenetic transmission have made them a research hotspot. Despite the important role of tRNA fragments in disease regulation, their biogenesis mechanism remains unclear. To address this, Jin Hanyong and his team focused on the IRE1α enzyme, a key regulatory factor in the cell's ER stress response. Under stress conditions, IRE1α selectively cleaves target gene RNA with a stem-loop structure and has been shown to regulate the unfolded protein response (UPR). Since tRNA possesses a stem-loop cloverleaf structure and certain specific tRNAs (such as GlyGCC and CysGCA) contain consensus sequences for IRE1α cleavage, the research team proposed a hypothesis: Can IRE1α generate functional tRNA fragments by cleaving specific tRNAs, and affect cell growth and adaptation under stress conditions?
The results show that, in various organisms, the IRE1α homolog selectively cleaves the anticodon loop of tRNAGly(GCC) at the same site under ER stress conditions, producing biologically functional 5′-tRH-GlyGCC fragments. This fragment regulates the alternative splicing of target gene mRNA in the nucleus by binding with the ribonucleoproteins HNRNPM and HNRNPH2, thereby promoting cancer cell proliferation. This mechanism demonstrates the conserved function of tRNA fragments in the cellular stress response, showing that they not only serve as biomarkers for stress but also play important roles in cellular adaptation and cancer growth.
This research achievement is the first Nature sub-journal article published by Yanbian University as the primary affiliation since its establishment. The study was conducted in collaboration with Chung-Ang University, NES Biotechnology, and Daegu Catholic University. The research was supported by the National Natural Science Foundation's Regional Fund Project and Yanbian University's “Tumen River Scholars Support Program.”
Nature Communications, launched in 2010 by Nature Publishing Group, is a premier multidisciplinary journal in the Nature family series dedicated to publishing significant, rigorous research advances across multiple scientific disciplines. With a 2024 impact factor of 14.7, it is classified as a Tier-1 journal in the Chinese Academy of Sciences ranking system.
Link to the paper:https://www.nature.com/articles/s41467-024-53624-4
First Review: He Xin | Second Review: Du You | Final Review: Chen Yanling
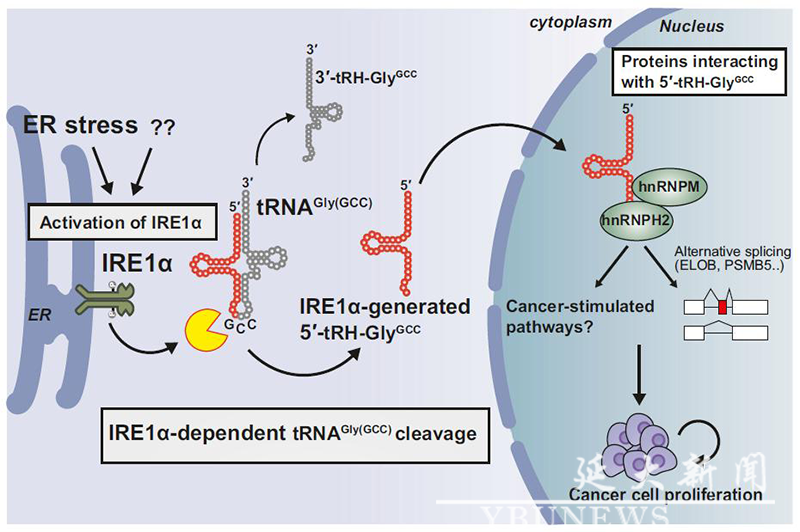
Proposed model for the IRE1α selective generation of 5′-tRH-GlyGCC that contributes to cellular adaptation upon ER stress presented in diverse eukaryotic organisms from yeast to humans.
